THE PROBLEM: BASIC WATER SCALING
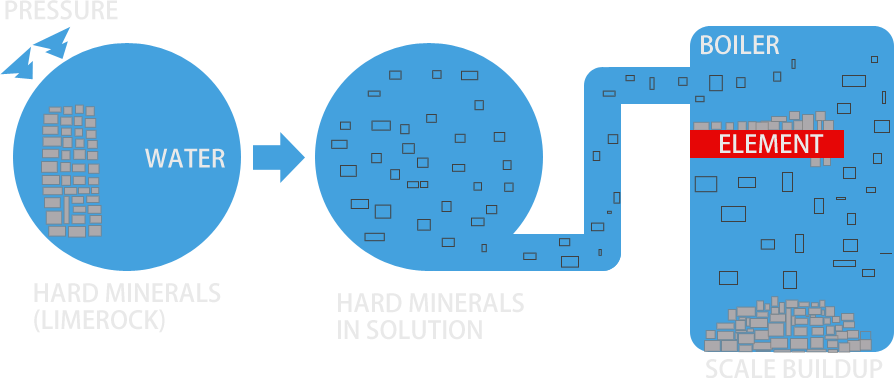
Water is the universal solvent. It dissolves everything it comes in contact with, whether it is as "hard" as iron or as "soft" as acid rain in the air. The water we use is saturated with many dissolved minerals. The amount of dissolved minerals depends on the water source and the water chemistry such as pH, oxygen content, temperature and pressure. When water is heated, its ability to hold calcium carbonate (scale) in solution is decreased. As temperature increases, calcium carbonate will begin to come out of solution and form hard water scale deposits. This causes harmful scale deposits in boilers, water heaters and other water fed equipment.

